So3 2 Estrutura De Lewis
Completo com fotos sobre So3 2 Estrutura De Lewis.
En ella dichos enlaces y los electrones se representan con puntos o guiones largos aunque la mayoría de las veces los puntos corresponden a los electrones no compartidos y los guiones a los enlaces covalentes. Wayne breslyn 625598 views.
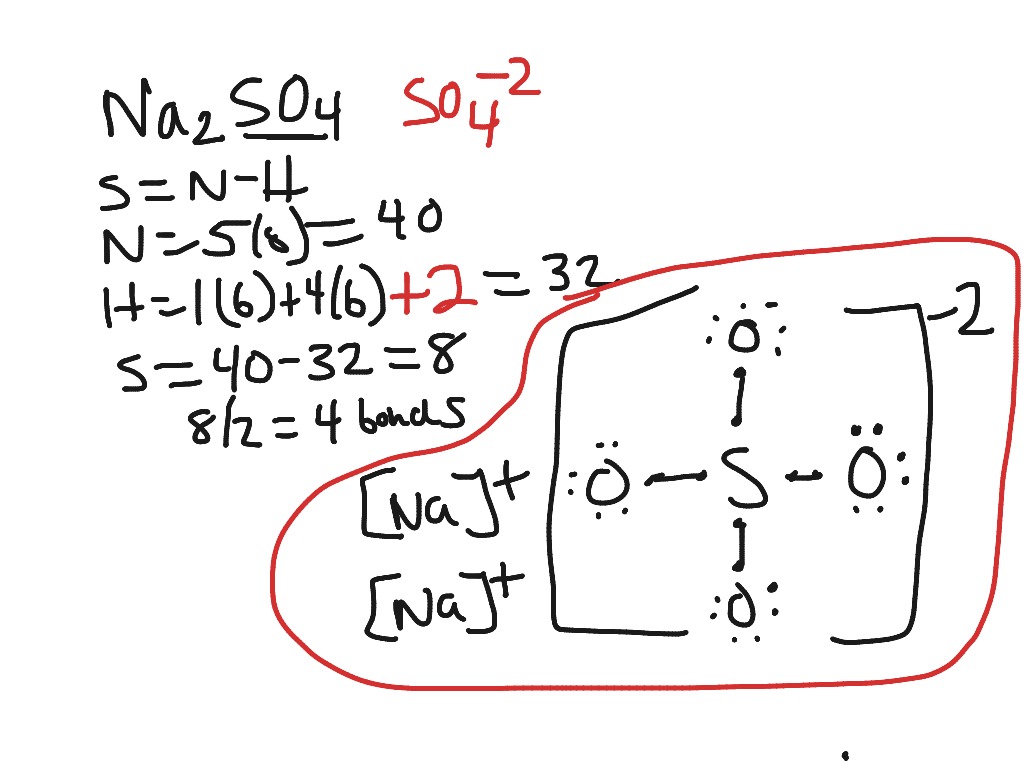
So4 2 lewis structure how to draw the lewis structure for so4 2.

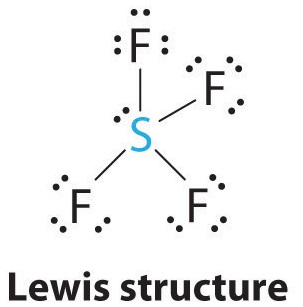
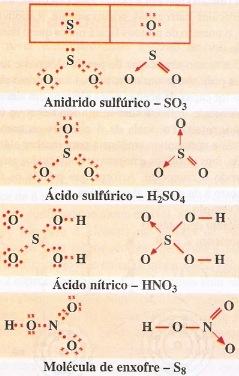
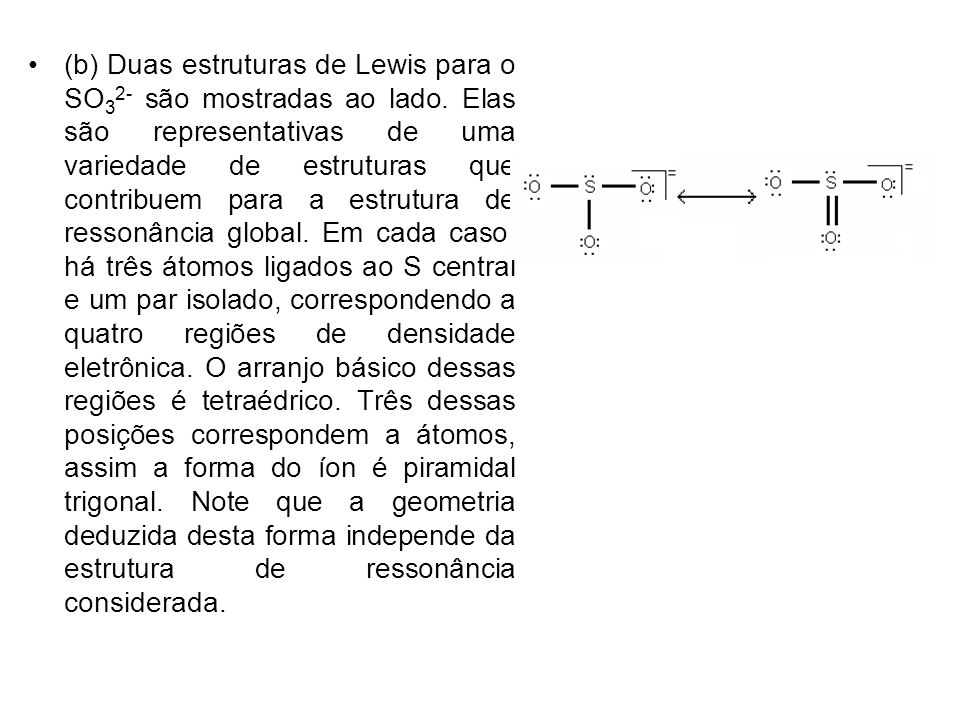
So3 2 estrutura de lewis. Lets do the so3 2 lewis structure. That also means that electrons are distributed differently. One pair of electrons. Lets put the sulfur at the center and the oxygens around the outside. La estructura de lewis es toda aquella representación de los enlaces covalentes dentro de una molécula o un ion. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons. So4 2 lewis structure how to draw the lewis structure for so4 2 sulfate ion duration. Several worked examples relevant to this procedure were given in previous posts please see the sitemap table of contents lewis electron dot structures. The chemical formula is so3 2. Let us consider the case of the sulfite ion. Put two electrons between the atoms to form chemical bonds. In mathmathrmso3math there are three double bonds and no lone pairs at sulfur in the hypervalent interpretation.
Examples and tricks for drawing lewis dot diagrams of molecules duration. Estrutura de lewis so4 2. A simple method for writing lewis structures is given. Alternatively you have one double bond. Lewis de aniones no2 no3 co32 so32 y so42 en este ejercicio determinaremos las estructuras de lewis de algunos aniones. So we have 26. Lewis structures made easy.
Encontre so3 2 estrutura de lewis aqui. Administrador blog Várias Estruturas 2020 compartilha informações e imagens relacionadas ao so3 2 estrutura de lewis que estamos procurando do compartilhamento de recursos.
Abaixo estão as fotos do so3 2 estrutura de lewis que o administrador Várias Estruturas blog 2020 coletou.






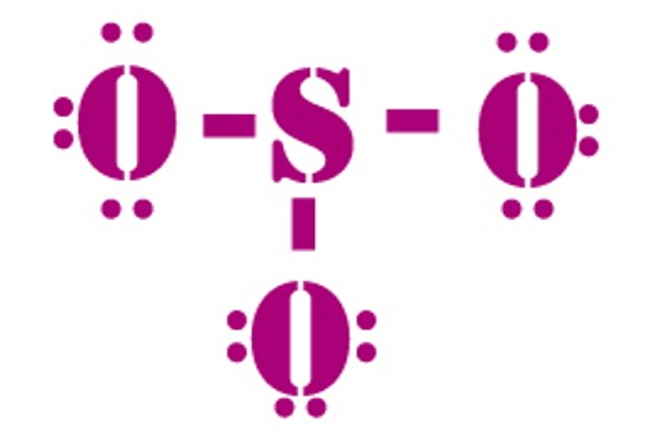




0 Response to "So3 2 Estrutura De Lewis"
Post a Comment